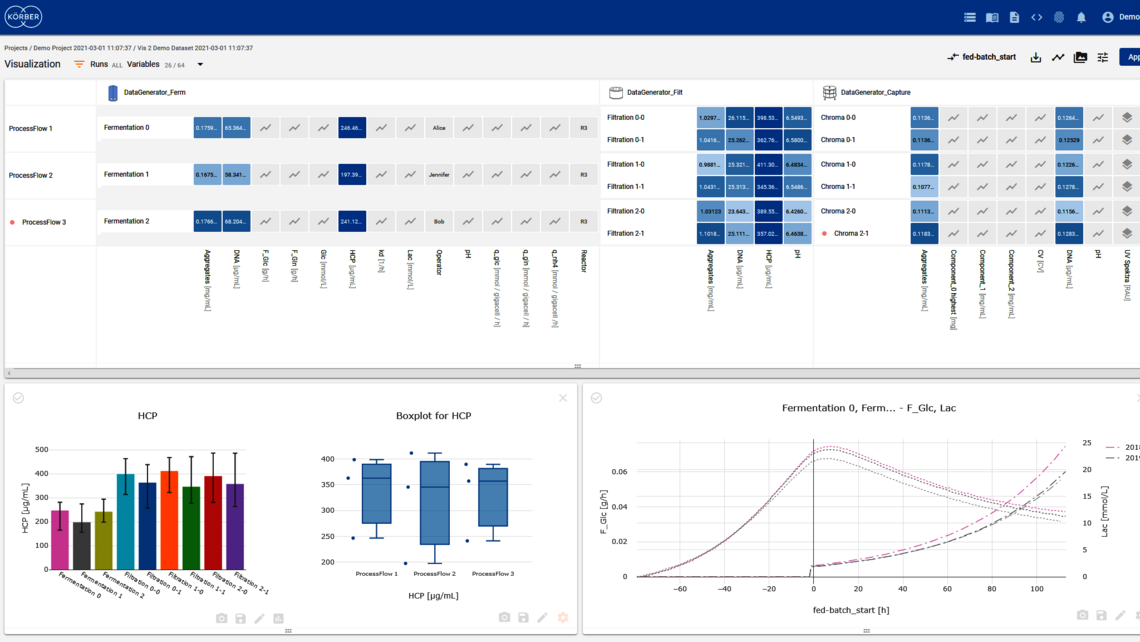
Over 60 percent of the world’s top 20 biotech manufacturers use PAS-X software to ensure flawless process execution, digital data management and fast batch review.
Biotech manufacturing creates specific challenges such as
- Complex processes with need for tight, real-time control
- Huge amounts of data that need to be analyzed
- Continuous manufacturing
- Loss of highly valuable batches must be prevented
Körber’s software solutions PAS-X MES and PAS-X Savvy support process control, enable real time control of the process, collect all exceptions that can be managed real time, and allow for direct intervention.